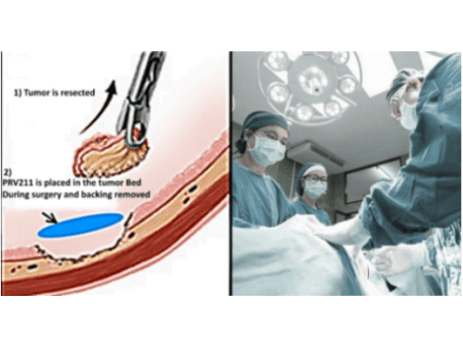
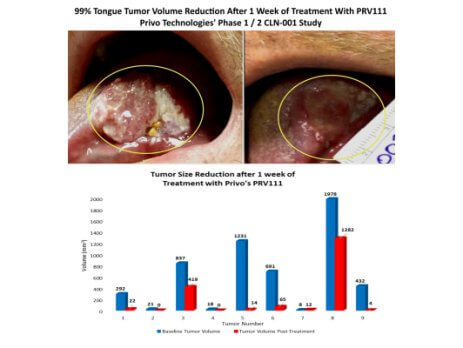
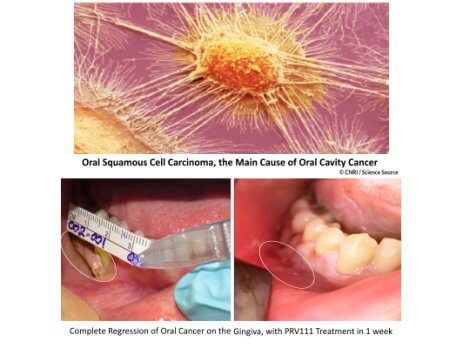
Privo Technologies, Inc. Receives IRB Approval for PRV211- Intraoperative Chemotherapy Treatment for Solid Tumors
Privo Technologies, Inc. is pleased to announce that it has received IRB approval for its CLN-004 study from WCG IRB. CLN-004 is a Phase 1/2, open-label, safety and efficacy study accessing the tolerability, anti-tumor effects, systemic exposure, and device...